Catalytic partial oxidation of methanol (POMeOH): addition of promoter gases into the feed.
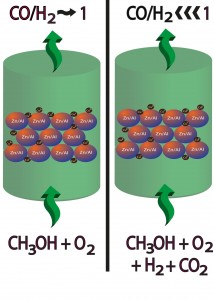
The addition of the reaction products (CO2 and H2) to the feed during the partial oxidation of methanol (POMeOH) over a Cu/ZnO/g-Al2O3 influences in a significantly way the selectivity to hydrogen during the reaction.
The observed changes are not due to the changes suggested by the thermodynamics when promoter gases are added and might be interpreted by considering modifications in the physicochemical properties of the catalysts, particularly in the kinetic of the reactions involved in POMeOH process.In some cases CO-free hydrogen (namely highly pure) could be obtained by POMeOH.
The processes consider the modulation of the selectivity by a controlled amount of CO2 and/or H2 into the reaction flux. The presence of promoter gases strongly influenced the Cu oxidation state. It is concludedthat the inhibition in CO formation is related to the high content of Cu0. It is suggested that in the presence of metallic copper the kinetic of the several reactions involved during POMeOH, facilitates the formation of H2 and CO2 and inhibits the CO formation. Results are useful for processes where the CO/H2 ratio has to be reduced drastically (fuel cells) or finely modulated (Fischer Tropsch, methanol synthesis, etc.).
_____________________________________________________________________________________________
Stability of Rh supported particles in methane dry reforming in membrane reactors
The stability of low Rh loading catalysts (0.2, 0.36 and 0.6% w/w, prepared from Rh(NO)3 precursor salt) supported on binary systems CaO-SiO2 with diffrent CaO % is evaluated in the reaction of dry reforming of methane in a conventional flow reactor at 550 ° C during one or more days. Stable catalysts (low Rh content) show high dispersion of Rh nanoparticles analyzed by HRTEM . However, catalysts with higher content of rhodium are very heterogeneous. Areas with a high density of Rh nanoparticles and other areas where these particles are detected in the HRTEM micrographs. Thus, the instability of these catalysts in the DRM reaction may be due to Rh particle agglomeration. This shows that the high dispersion of metal particles is to maintain the stability of the catalyst, since a significant metal agglomeration could cause deactivation.
 Los contenidos de esta web están sujetos a una licencia
Los contenidos de esta web están sujetos a una licencia